Abstract
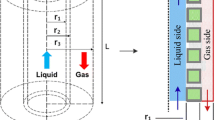
In this paper a 3-dimensional modeling of simultaneous stripping of carbon dioxide (CO2) and hydrogen sulfide (H2S) from water using hollow fiber membrane made of polyvinylidene fluoride is developed. The water, containing CO2 and H2S enters to the membrane as feed. At the same time, pure nitrogen flow in the shell side of a shell and tube hollow fiber as the solvent. In the previous methods of modeling hollow fiber membranes just one of the membranes was modeled and the results expand to whole shell and tube system. In this research the whole hollow fiber shell and tube module is modeled to reduce the errors. Simulation results showed that increasing the velocity of solvent flow and decreasing the velocity of the feed are leads to increase in the system yield. However the effect of the feed velocity on the process is likely more than the influence of changing the velocity of the gaseous solvent. In addition H2S stripping has higher yield in comparison with CO2 stripping. This model is compared to the previous modeling methods and shows that the new model is more accurate. Finally, the effect of feed temperature is studied using response surface method and the operating conditions of feed temperature, feed velocity, and solvent velocity is optimized according to synergistic effects. Simulation results show that, in the optimum operating conditions the removal percentage of H2S and CO2 are 27 and 21 % respectively.
















Similar content being viewed by others
Abbreviations
- I:
-
I-component (CO2 and H2S)
- C0 :
-
Inlet mixture concentration (mol/m3)
- C:
-
Concentration (mol/m3)
- Ci-membrane :
-
Component i concentration in the membrane (mol/m3)
- Ci-shell :
-
Component i concentration in the shell (mol/m3)
- Ci-tube :
-
Component i concentration in the tube (mol/m3)
- D:
-
Diffusion coefficient (m2/s)
- Di-membrane :
-
Diffusion coefficient of component i in the membrane (m2/s)
- Di-shell :
-
Diffusion coefficient of component i in the shell (m2/s)
- Di-tube :
-
Diffusion coefficient of component i in the tube (m2/s)
- F:
-
Body force (N)
- Ji :
-
Diffusive flux of species i (mol/s)
- m:
-
Physical solubility (dimensionless)
- r:
-
Radial coordinate (m)
- r1 :
-
Inner tube radius (m)
- r-2 :
-
Outer tube radius (m)
- r-3 :
-
Inner shell radius (m)
- Ri :
-
Reaction rate of species i (mol/s)
- t:
-
Time (s)
- u:
-
Average velocity in the tube side (m/s)
- V:
-
Velocity vector (m/s)
- Vz :
-
z Direction velocity in the contactor (m/s)
- Vz-shell :
-
z Direction velocity in the shell (m/s)
- ∇:
-
Gradient (dimensionless)
- ρ:
-
Density (kg/m3)
- η:
-
Dynamic viscosity (m2/s)
- in:
-
Inlet
- out:
-
Outlet
- Vz-tube :
-
z Direction velocity in the tube (m/s)
- Vinlet :
-
Inlet velocity to the contactor (m/s)
- z:
-
Axial distance (m)
References
Faiz R, Al-Marzouqi M (2009) Mathematical modeling for the simultaneous absorption of CO2 and H2S using MEA in hollow fiber membrane contactors. J Membr Sci 342:269–278. doi:10.1016/j.memsci.2009.06.050
Al-Wohoush M, Van Heiningen A, Kubes G (1996) Selective absorption of H2S from a CO2 containing gas in a solution of sodium carbonate. In: AIChE symposium series, vol 311. American institute of chemical engineers, pp 106–112
Wallin M, Olausson S (1993) Simultaneous absorption of H2S and CO2 into a solution of sodium carbonate. Chem Eng Commun 123(1):43–59
Mansourizadeh A, Ismail AF (2011) CO2 stripping from water through porous PVDF hollow fiber membrane contactor. Desalination 273(2–3):386–390. doi:10.1016/j.desal.2011.01.055
Atchariyawut S, Feng C, Wang R, Jiraratananon R, Liang D (2006) Effect of membrane structure on mass-transfer in the membrane gas–liquid contacting process using microporous PVDF hollow fibers. J Membr Sci 285(1):272–281
Das A, Abou-Nemeh I, Chandra S, Sirkar KK (1998) Membrane-moderated stripping process for removing VOCs from water in a composite hollow fiber module. J Membr Sci 148(2):257–271. doi:10.1016/S0376-7388(98)00167-7
deMontigny D, Tontiwachwuthikul P, Chakma A (2006) Using polypropylene and polytetrafluoroethylene membranes in a membrane contactor for CO <sub>2</sub> absorption. J Membr Sci 277(1):99–107
Estay H, Bocquet S, Romero J, Sanchez J, Rios GM, Valenzuela F (2007) Modeling and simulation of mass transfer in near-critical extraction using a hollow fiber membrane contactor. Chem Eng Sci 62(21):5794–5808. doi:10.1016/j.ces.2007.05.037
Gabelman A, Hwang S-T (1999) Hollow fiber membrane contactors. J Membr Sci 159(1–2):61–106. doi:10.1016/S0376-7388(99)00040-X
Ismail AF, Mansourizadeh A (2010) A comparative study on the structure and performance of porous polyvinylidene fluoride and polysulfone hollow fiber membranes for CO <sub>2</sub> absorption. J Membr Sci 365(1):319–328
Kiani A, Bhave RR, Sirkar KK (1984) Solvent extraction with immobilized interfaces in a microporous hydrophobic membrane. J Membr Sci 20(2):125–145. doi:10.1016/S0376-7388(00)81328-9
Lu J-G, Zheng Y-F, Cheng M-D (2009) Membrane contactor for CO <sub>2</sub> absorption applying amino-acid salt solutions. Desalination 249(2):498–502
Mansourizadeh A, Ismail A, Matsuura T (2010) Effect of operating conditions on the physical and chemical CO <sub>2</sub> absorption through the PVDF hollow fiber membrane contactor. J Membr Sci 353(1):192–200
Marzouk SA, Al-Marzouqi MH, El-Naas MH, Abdullatif N, Ismail ZM (2010) Removal of carbon dioxide from pressurized CO <sub>2</sub>–CH <sub>4</sub> gas mixture using hollow fiber membrane contactors. J Membr Sci 351(1):21–27
Prasad R, Khare S, Sengupta A, Sirkar K (1990) Novel liquid-in-pore configurations in membrane solvent extraction. AIChE J 36(10):1592–1596
Prasad R, Sirkar K (2004) Dispersion-free solvent extraction with microporous hollow-fiber modules. AIChE J 34(2):177–188
Ravanchi MT, Kaghazchi T, Kargari A (2010) Facilitated transport separation of propylene–propane: experimental and modeling study. Chem Eng Process 49(3):235–244. doi:10.1016/j.cep.2010.01.011
Xu A, Yang A, Young S, demontigny D, Tontiwachwuthikul P (2008) Effect of internal coagulant on effectiveness of polyvinylidene fluoride membrane for carbon dioxide separation and absorption. J Membr Sci 311(1):153–158
Bounaceur R, Castel C, Rode S, Roizard D, Favre E (2012) Membrane contactors for intensified post combustion carbon dioxide capture by gas–liquid absorption in MEA: a parametric study. Chem Eng Res Des 90(12):2325–2337. doi:10.1016/j.cherd.2012.05.004
Dindore VY, Cents AHG, Brilman DWF, Versteeg GF (2005) Shell-side dispersion coefficients in a rectangular cross-flow hollow fibre membrane module. Chem Eng Res Des 83(3):317–325. doi:10.1205/cherd.04166
Ettouney HM, El-Dessouky HT, Abou Waar W (1999) Separation characteristics of ternary gas mixtures in serial cells of polysulfone hollow fibre membranes. Chem Eng Res Des 77(5):395–404. doi:10.1205/026387699526377
Khalilpour R, Abbas A, Lai Z, Pinnau I (2013) Analysis of hollow fibre membrane systems for multicomponent gas separation. Chem Eng Res Des 91(2):332–347. doi:10.1016/j.cherd.2012.07.009
Vernekar PV, Jagdale YD, Patwardhan AW, Patwardhan AV, Ansari SA, Mohapatra PK, Manchanda VK (2013) Transport of cobalt(II) through a hollow fiber supported liquid membrane containing di-(2-ethylhexyl) phosphoric acid (D2EHPA) as the carrier. Chem Eng Res Des 91(1):141–157. doi:10.1016/j.cherd.2012.06.019
Afrane G, Chimowitz EH (1996) Experimental investigation of a new supercritical fluid-inorganic membrane separation process. J Membr Sci 116(2):293–299. doi:10.1016/0376-7388(96)00049-X
Chiu Y-W, Tan C-S (2001) Regeneration of supercritical carbon dioxide by membrane at near critical conditions. J Supercrit Fluids 21(1):81–89. doi:10.1016/S0896-8446(01)00074-2
Happel J (2004) Viscous flow relative to arrays of cylinders. AIChE J 5(2):174–177
Khelifa A, Moulay S, Hannane F, Benslimene S, Hecini M (2004) Application of an experimental design method to study the performance of electrochlorination cells. Desalination 160(1):91–98. doi:10.1016/S0011-9164(04)90021-5
Mansourizadeh A (2012) Experimental study of CO <sub>2</sub> absorption/stripping via PVDF hollow fiber membrane contactor. Chem Eng Res Des 90(4):555–562
Versteeg GF, Van Swaalj W (1988) Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkanolamine solutions. J Chem Eng Data 33(1):29–34
Dindore V, Brilman D, Versteeg G (2005) Modelling of cross-flow membrane contactors: mass transfer with chemical reactions. J Membr Sci 255(1):275–289
Cussler EL (1997) Diffusion: mass transfer in fluid systems. Cambridge university press, Cambridge
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Solubility
The distribution coefficient of CO2 in pure water is taken from Versteeg and van Swaaij [29]. Also the distribution coefficient of CO2 in pure water was taken from Dindore et al. [30].
where
1.2 Diffusivity
The diffusivity of CO2 in pure water was taken from Versteeg and van Swaaij [29]. The diffusivity of H2S in pure water was taken from Cussler and Edvard [31].
The diffusivity of CO2 in N2 can be calculated based on Chapman–Enskog theory [31].
Rights and permissions
About this article
Cite this article
Bahlake, A., Farivar, F. & Dabir, B. New 3-dimensional CFD modeling of CO2 and H2S simultaneous stripping from water within PVDF hollow fiber membrane contactor. Heat Mass Transfer 52, 1295–1304 (2016). https://doi.org/10.1007/s00231-015-1635-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1635-y